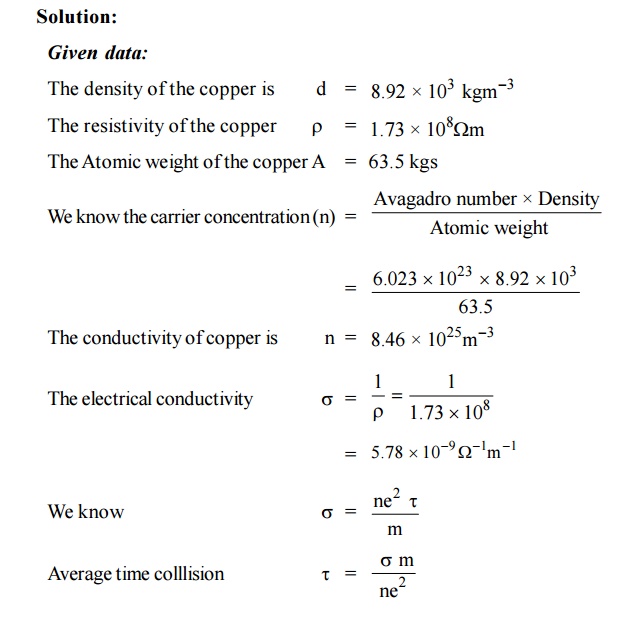
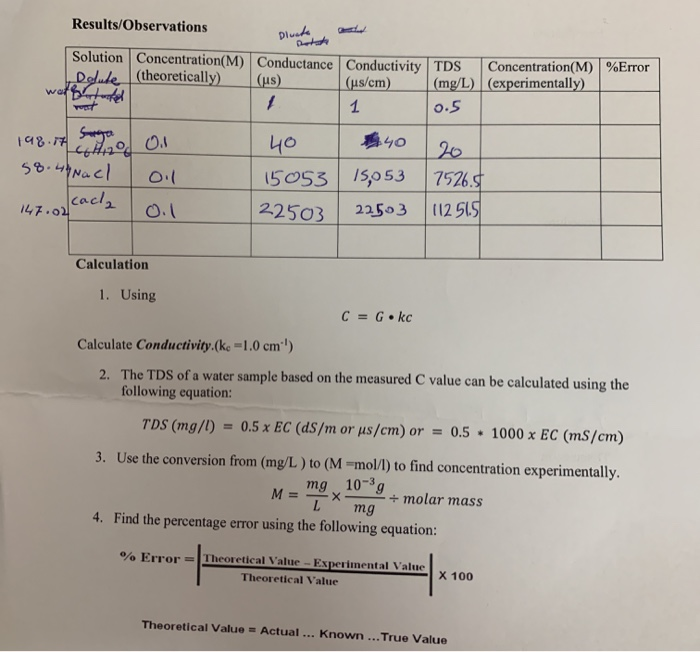
The conductivity of `0.1`m KCl solution is `1.29sm^(-1)`. If the resistance of the cell filled with - YouTube

The specific conductivity of a solution containing `1.0g` of anhydrous `BaCI_(2)` in `200 cm^(3)` of - YouTube
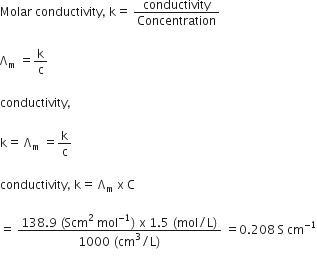
The molar conductivity of a 1.5 M solution of an electrolyte is found to be 138.9S cm2mol-1. Calculate the conductivity of the conductivity of this solution. from Chemistry Electrochemistry Class 12 UP Board
The conductivity of saturated solution of silver chloride is 1.24×10^6 S/cm. The ionic conductivity of Ag+ and Cl at infinite dilution are 53.8 and 65.3 Scmsq/mol. Calculate the solubility of AgCl in

Molar conductivity of 0.02 M HCl solution is 407.2 Ω^-1 cm^2 mol^-1 at 25^∘C . Calculate its conductivity.?

The molar conductivity of a 1.5M solution of an electrolyte is found to be 138.9s cm^2 mol^-1 . Calculate the conductivity of this solution.

The molar conductivity of a 1.5 M solution of an electrolyte is found to be `138.9 S cm^(2) mol^(-1) - YouTube

Calculate the molar conductivity of a solution of NaCl at infinite dilution. Given lambda^(oo)(Na^(+))=50.11 "ohm"^(-1)cm^(2)mol^(-1) lambda^(oo)(Cl^(-))=76.34 "ohm"^(-1)cm^(2)mol^(-1)
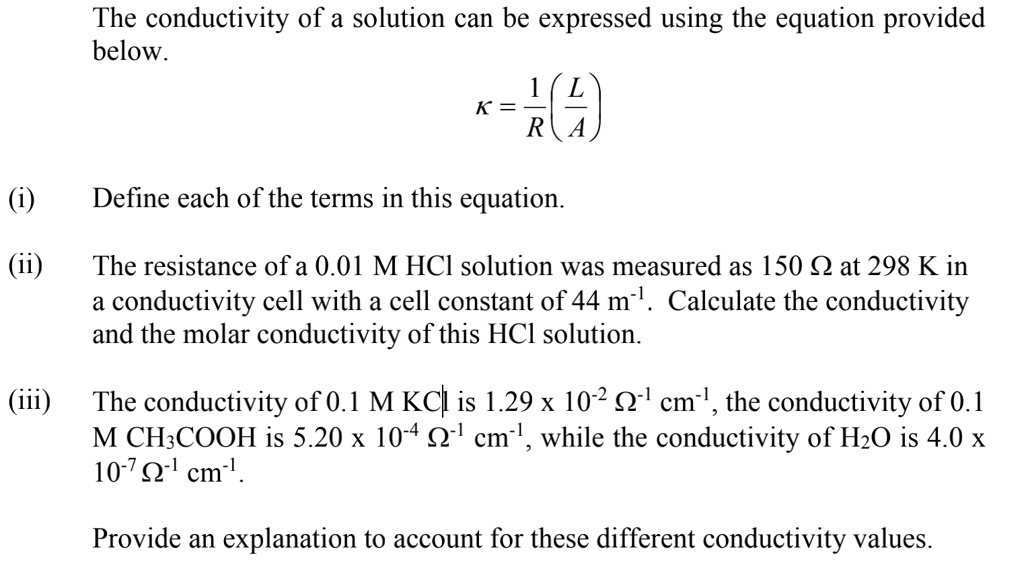
SOLVED: The conductivity of a solution can be expressed using the equation provided below. K = R(4) R Define each of the terms in this equation. (ii) The resistance of a 0.01