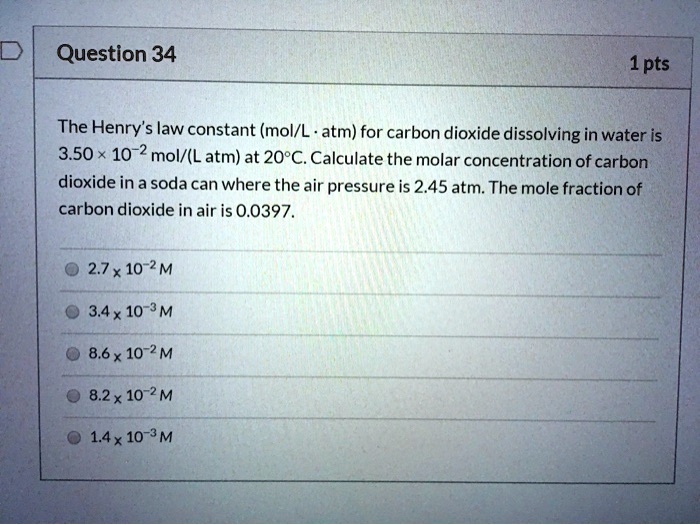
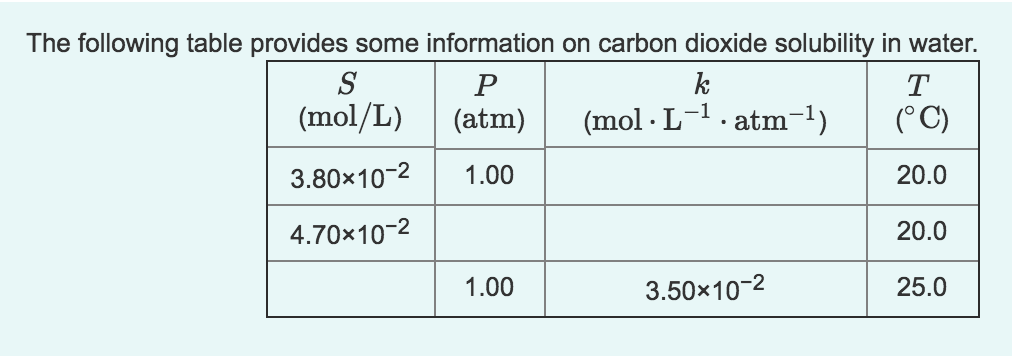
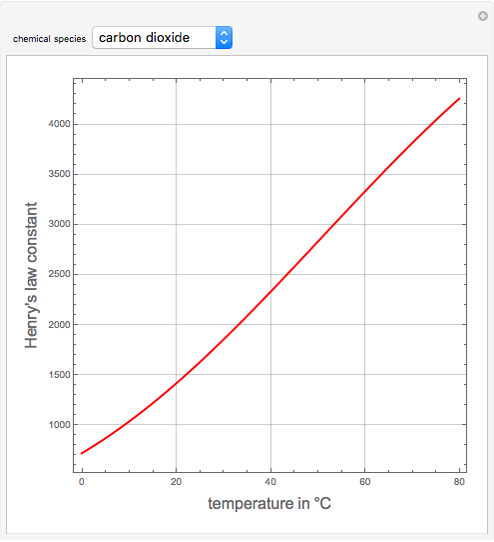
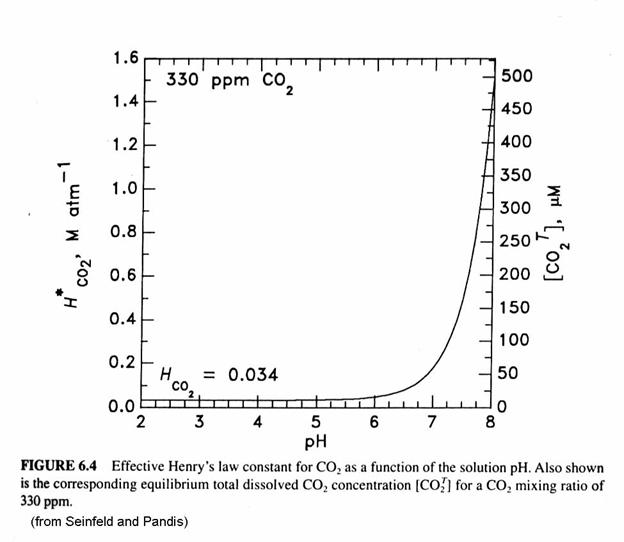
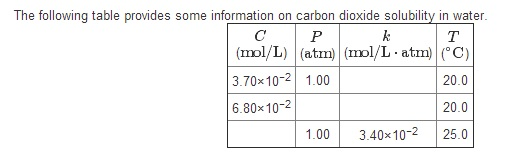
2.7 Henry's law constant for CO, in water is 1.67x108 Pa at 298 K. Calculate2the quantity of CO, in 500 mL of soda water when packed under 2.5 atmCO2 pressure at 298 K.
![Henry's law constant for CO(2) in water is 2.5xx10^(8)Pa at 298K. Calculate mmole of CO(2) dissolved in 14g water at 2.5atm pressure at 298K. [Take 1atm=10^(5)N//m^(2) or Pa] Henry's law constant for CO(2) in water is 2.5xx10^(8)Pa at 298K. Calculate mmole of CO(2) dissolved in 14g water at 2.5atm pressure at 298K. [Take 1atm=10^(5)N//m^(2) or Pa]](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/16113056_web.png)
Henry's law constant for CO(2) in water is 2.5xx10^(8)Pa at 298K. Calculate mmole of CO(2) dissolved in 14g water at 2.5atm pressure at 298K. [Take 1atm=10^(5)N//m^(2) or Pa]
Henry's law constant for CO2 in water is 1.67 x 10^8 Pa at 298 K. - Sarthaks eConnect | Largest Online Education Community
Henry's law constant for CO2 in water is 1.67 × 108 Pa at 298 K. Calculate the quantity - Sarthaks eConnect | Largest Online Education Community
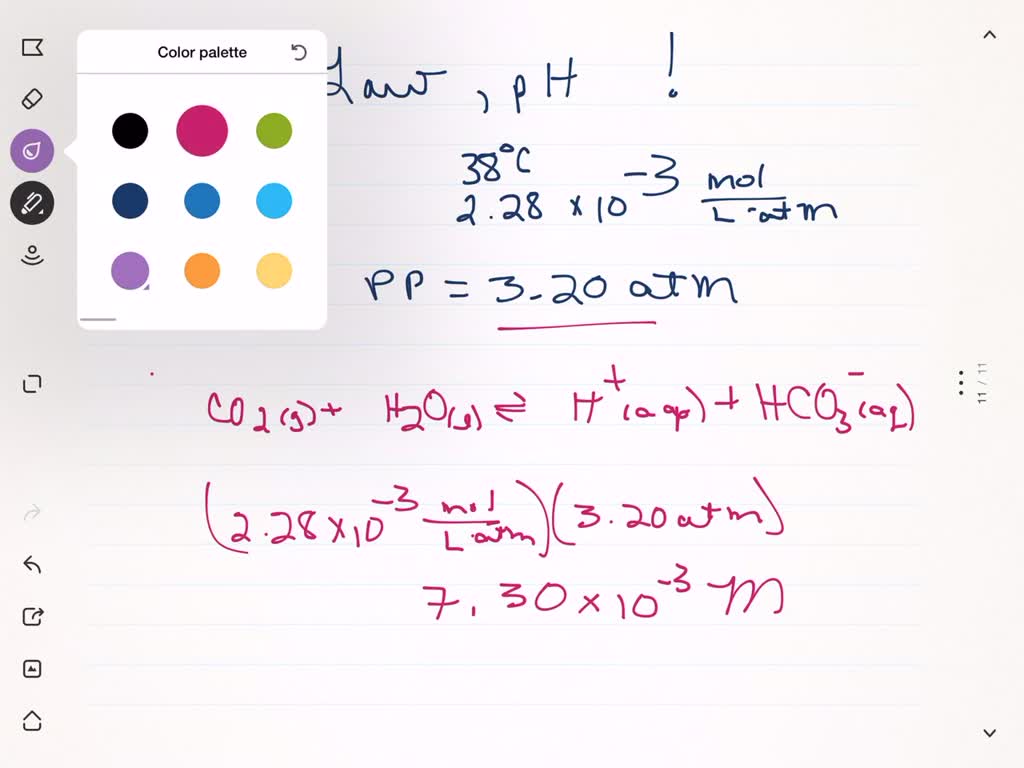
SOLVED:Henry's law constant for CO2 at 38^∘ C is 2.28 ×10^-3 mol / L ·atm. Calculate the pH of a solution of CO2 at 38^∘ C in equilibrium with the gas at

Natural Logarithm of Henry's Law Constant for the Adsorption of CH 4 in... | Download Scientific Diagram
Henry's law constant for CO2 in water is 1.67 × 108 Pa at 298 K. Calculate the quantity - Sarthaks eConnect | Largest Online Education Community

Henry's law constant for CO2 in water is 1.67 X 10^8 Pa at 298K. Calculate the quantity of CO2 in... - YouTube
Henry's law constant of CO2 in water at 298 K is 5/3 K bar. Determine its concentration (mole fraction) in rain if CO2 is 1

Henry's law constants and diffusivities for gases in water at 37 °C a . | Download Scientific Diagram

Henry's law constant for CO2 in water is 1.67 × 10^8 Pa at 298K. Calculate the quantity of CO2 in 500 ml of soda water when packed under 2.5 atm CO2 pressure at 298 K.
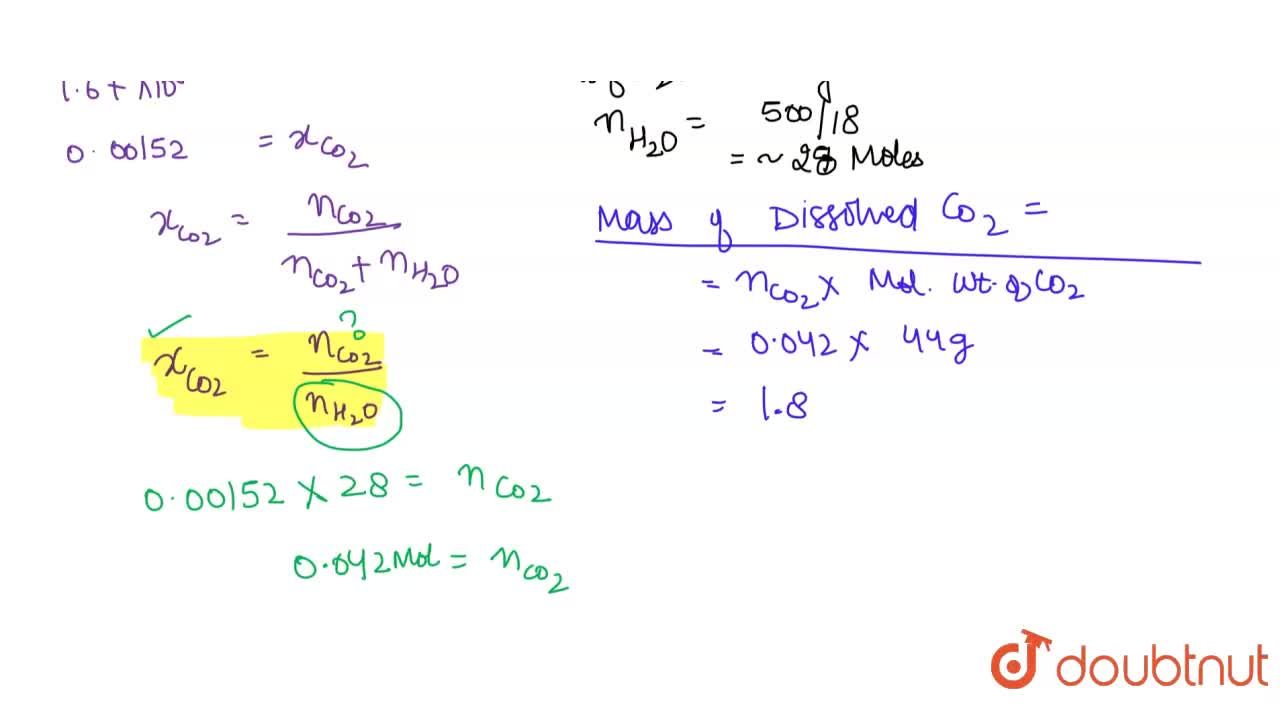
Henry's law constant for CO(2) in water is 1.67xx10^(8) Pa at 298 K. Calculate the quantity of CO(2) in 500mL of soda water when packed under 2.5atm CO(2) pressure at 298 K.