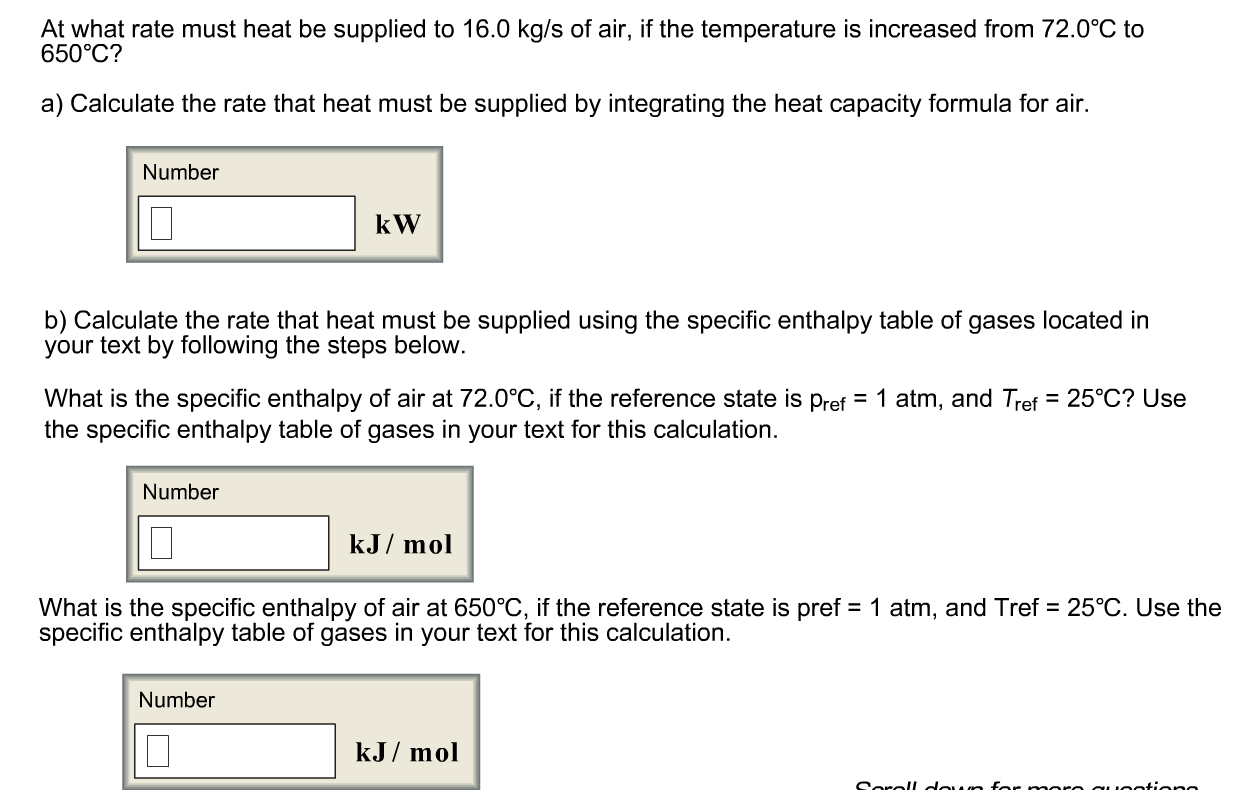
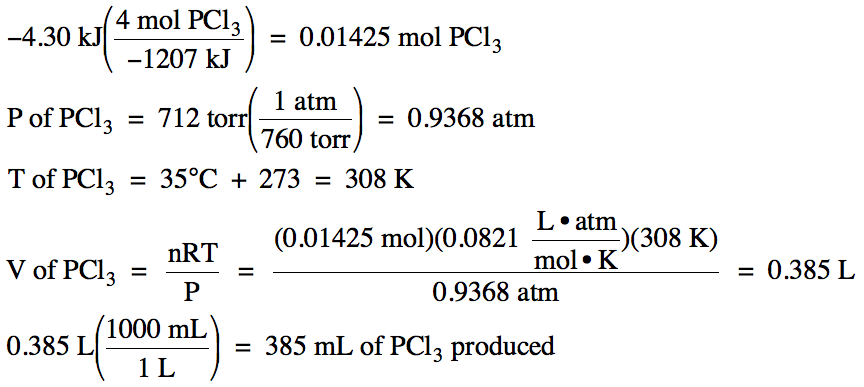
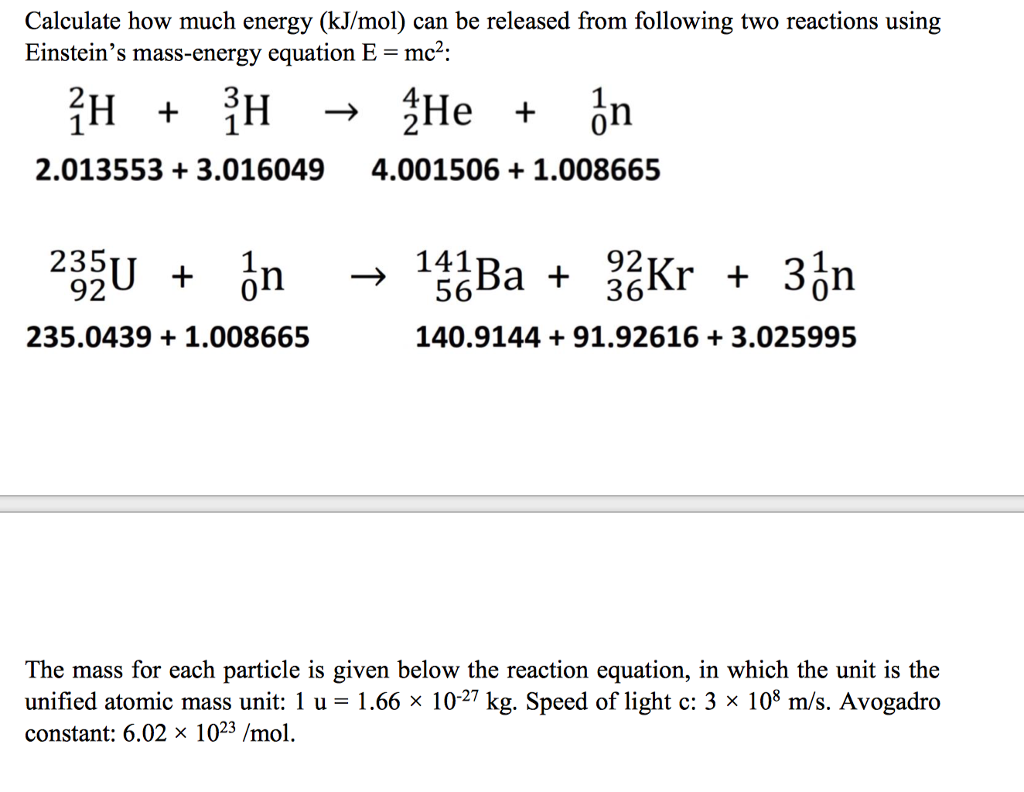
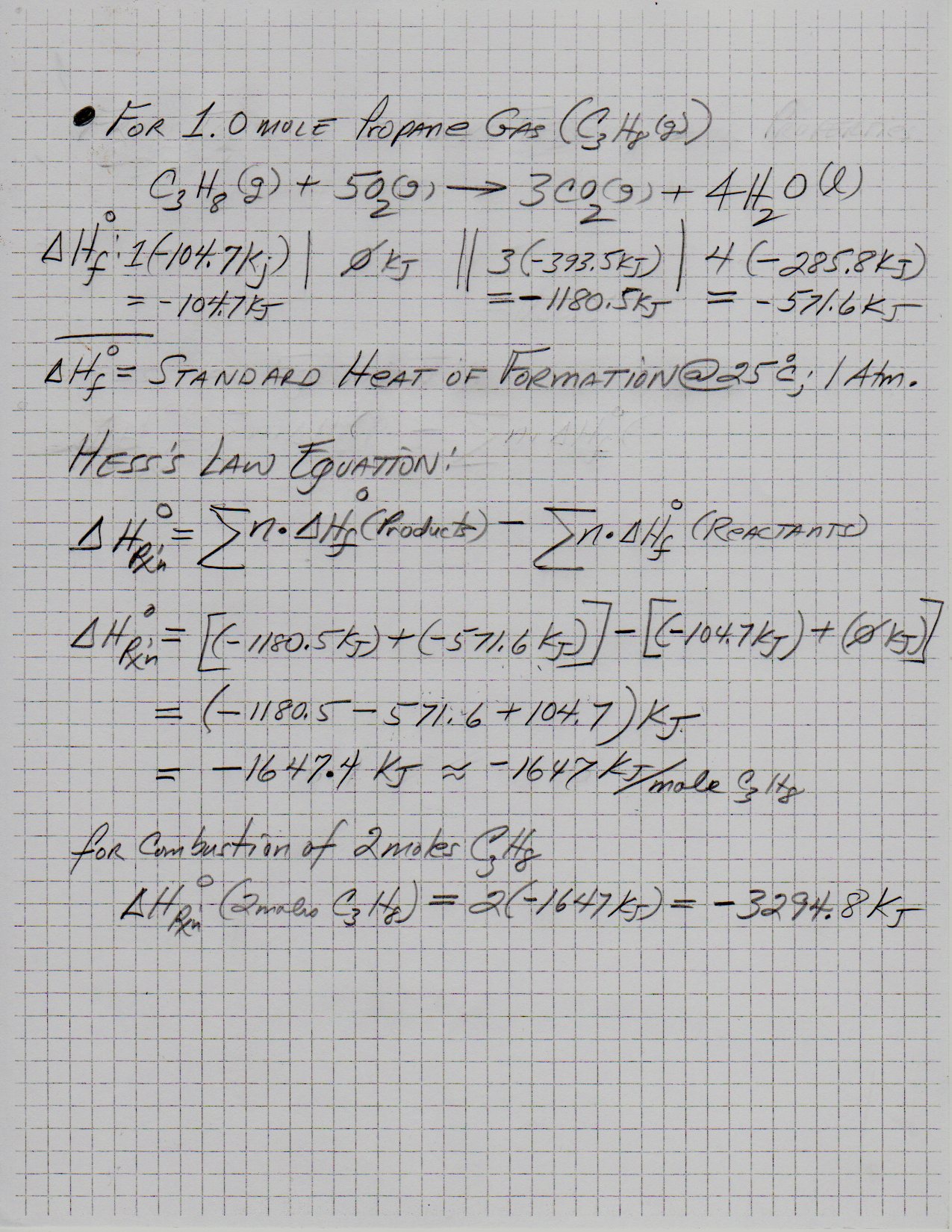Calculate the calorific value of methane if it burns according to the equation CH4(g) + 2O2(g) to CO2(g) + 2H2O(l) , DeltaH = -890.0 kJ
The heat of combustion of C, H2 and CH4 at 298 K and 1 atm are respectively-393 kJ/mol, -286 kJ/mol, and -892 kJ/mol. How do I calculate the enthalpy of formation for

Homework 3 Solutions - Introductory Physical Chemistry | CH 331 | Assignments Physical Chemistry | Docsity